Abstract
Introduction: Recent data suggest a suboptimal antibody response to COVID-19 vaccination in patients with multiple myeloma (MM), especially under treatment. Herein, we evaluated the development of neutralizing antibodies (NAbs) against SARS-CoV-2 in non-vaccinated MM patients who were diagnosed with COVID-19 compared to MM patients after full vaccination with the mRNA BNT162b2 vaccine.
Methods: The analysis was performed in the context of an ongoing large prospective study (NCT04743388) evaluating the kinetics of anti-SARS-CoV-2 antibodies after COVID-19 vaccination. We evaluated MM patients diagnosed with COVID-19, confirmed by PCR, matched for age, gender, line of treatment, type of myeloma, type of treatment and response with vaccinated MM patients during the same time period (January - May 2021). Major exclusion criteria for both COVID-19 and vaccine MM groups included the presence of: (i) autoimmune disorder under immunosuppressive therapy or other active cancer; (ii) active HIV, hepatitis B and C infection, and (iii) end-stage renal disease . Serum was collected at 4 th week post confirmed diagnosis for the COVID-19 MM group and at 4 th week post the second BNT162b2 dose for the vaccine MM group. NAbs against SARS-CoV-2 were measured using an FDA approved methodology (cPass™ SARS-CoV-2 NAbs Detection Kit, GenScript, Piscataway, NJ, USA).
Results: We evaluated 35 patients with MM and COVID-19 (6 had smoldering MM and 29 symptomatic MM), along with 35 matched MM patients who received the BNT162b2 vaccine.
Among COVID-19 MM patients, 13 were diagnosed with mild, 12 with moderate and 10 with severe disease; 22/35 patients were hospitalized and 10/35 were intubated. Seven (20%) patients died due to COVID-19. During the disease course 21 patients (60%) were treated with dexamethasone. Type of treatment was not different between COVID-19 positive and vaccinated MM patients. Between the two patient groups, there was no difference in terms of age [median (IQR) 65 (59) for COVID-19 positive versus 66 (74) for COVID-19 vaccinated, respectively, p=0.76], gender [males: 19/35 (54.3%) versus 16/35 (45.7%), respectively, p=0.47), BMI (median 27 versus 26kg/m 2, respectively, p=0.56), asymptomatic disease [6/35 (18.2%) in both groups, p=1], prior lines of treatment [range: 1 to 7 versus 1 to 6, respectively, p=0.99], and type of treatment (p=0.87). Among the COVID-19 MM patients, 6 (20.7%) were in sCR/CR, 6 (20.7%) in VGPR, 12 (41.4%) patients in PR, 2 (6.9%) in MR/SD and one (3.5%) in PD at the time of confirmed infection. Among the vaccinated MM group, 10 (34.5%) patientswere in sCR/CR, 4 (13.8%) in VGPR, 11 (37.9%) in PR, one (3.5%) in MR/SD and one (3.5%) in PD at the time of vaccination (p-value=0.93 for the comparison between COVID-19 and vaccinated MM groups). No differences between COVID-19 and vaccinated MM patients were also noted regarding the median lymphocyte count (1200/μl versus 1400/μl, respectively, p=0.08) and the median immunoglobulin values (IgG 732 mg/dl versus 747 mg/dl, respectively, p=0.29; IgA 9 mg/dl versus 61 mg/dl, respectively, p=0.7; IgM 26 mg/dl versus 25 mg/dl, p=0.97). The incidence of comorbidities was also similar between the two groups (cardiovascular diseases 55.2% versus 44.8%, respectively, p=0.47; diabetes mellitus 66.7% versus 33.3%, p=0.28; chronic pulmonary disease 50% each, p=1.0).
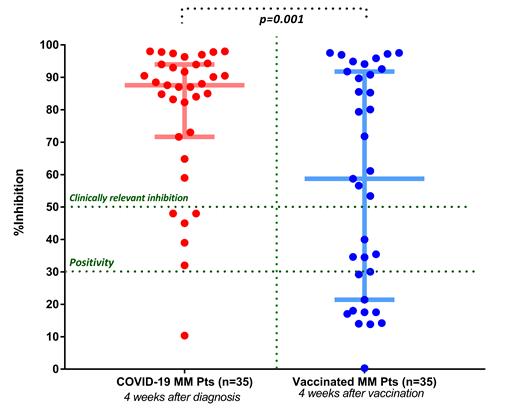
Interestingly, patients with MM and COVID-19 showed a superior humoral response compared with vaccinated MM patients. The median (IQR) NAb titers were 87.6% (IQR: 71.6-94) and 58.7% (21.4-91.8) for COVID-19 and for vaccinated MM patients, respectively (p=0.01). In both groups, 27 out of 35 patients were receiving active treatment for MM at the time of NAb evaluation. The median NAb titer was 88% (IQR 71.6%-96.3%) for COVID-19 MM patients and 35.4% (IQR 17.5%-85.5%) for vaccinated MM patients who received anti-myeloma therapy (p=0.001). Importantly, there was no difference in NAb production between COVID-19 and vaccinated MM patients who did not receive any treatment (median NAb titers, 85.1% versus 91.7%, p=0.14).
Conclusion: Patients with MM and COVID-19 present a superior NAb response against SARS-CoV-2 compared with fully vaccinated patients with the BNT162b2 vaccine. This finding was more pronounced among patients receiving active treatment for MM. In this context, additional booster doses may be considered for MM patients with poor humoral response after the BNT162b2 vaccine.
Gavriatopoulou: Genesis: Honoraria; Karyopharm: Honoraria; Takeda: Honoraria; Janssen: Honoraria; Sanofi: Honoraria; GSK: Honoraria; Amgen: Honoraria. Terpos: BMS: Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Janssen-Cilag: Consultancy, Honoraria, Research Funding; GSK: Honoraria, Research Funding; Novartis: Honoraria; Genesis: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding. Kastritis: Amgen: Consultancy, Honoraria, Research Funding; Genesis Pharma: Honoraria; Janssen: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Takeda: Honoraria. Dimopoulos: Amgen: Honoraria; BMS: Honoraria; Janssen: Honoraria; Takeda: Honoraria; BeiGene: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal